|
Photos
Respond to this story
|
Mayo Clinic tests new smallpox vaccine
December 13, 2002
President Bush today ordered smallpox shots for military personnel, and recommended immunization for certain health care and emergency workers. The President did not recommend immunizations for anyone else --- unless there is a biological attack. In Rochester, Mayo Clinic staff are currently working on what they believe may be a new safer smallpox vaccination. They are the only researchers in the US conducting tests on humans.
Rochester, Minn. — Healthy adults between the ages of 18 and 29 have been steadily enrolling in a Mayo Clinic study, designed to test the effectiveness of a promising new smallpox vaccination.
The US had a smallpox immunization program until 1972. But study participants are all young enough to have missed those vaccinations. Volunteers receive either the old original smallpox vaccine or some variation of the new version.
 | |||
Greg Poland heads Mayo's Vaccine Research Group and is the study's lead investigator. Poland says researchers think the new version will have fewer side effects because of how it's made.
"The currently licensed vaccine is made by taking the virus and then scratching into the skin of calves," he says. "Then the lymph fluid and tissue fluid leaks out and it gets collected and purified and made into a vaccine. The new vaccine that we're using is made a lot differently than that. We use the same strain of virus, but that virus is used to infect human cells that are in sterile cultures. "
Using this human cell based vaccine could stem some of the more dangerous side effects. For example one in a million people will die as a result of the vaccine. While 15 people out a million will develop a life threatening illness.
 | |||
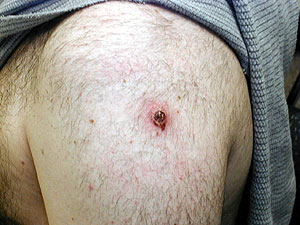
David Schnepple is a Mayo researcher participating in the study. He was vaccinated at the end of November. Schnepple puts on a pair of plastic surgical gloves, and slowly peals off a bandage on his upper arm.
"You can see its scabbed over now, its about the size of a pencil eraser tip, maybe larger" Schnepple says. "I don't feel any pain at the site, but its still red and looks scabbed," says Schnepple.
The vaccine is administered by a two-pronged needle. It breaks the surface the skin 15 times. In most cases there is no immediate physical reaction.
For Schnepple it was 6 days before fluid started to build and the scab developed. But he's not out of the woods yet. He's still potentially infectious. Schnepple must keep the site carefully covered until the scab falls off.
"Until the scab separates potentially you would touch the vaccine site and scratch your own skin and you would develop a pock," says Schnepple.
That makes the vaccine especially dangerous for people with compromised immune systems, pregnant women, and infants.
Mayo's testing the vaccine for the pharmaceutical company Acampis and the CDC.
Mayo's Greg Poland says if the study lives up to expectations, the new vaccine could sail through the FDA approval process and be available to the public by 2004.
"The advantage to the American," Poland says, "Is the notion or idea that in a shorter period of time we could have new vaccine licenses. Typically it takes anywhere to from 10 to 20 years to license a vaccine. This one maybe licensed in two to three years."
Mayo is still accepting participants for this leg of the study. In February, the clinic will launch a closely related study using older participants who will be re-immunized against the disease. Researchers say it's unclear whether adults who received smallpox vaccinations as children are still immune. It's likely, an additional vaccination will be recommended.
|
News Headlines
|
Related Subjects
|